1512 月 2016
The new model, KP-B210 obtain FDA 510k
The new model, KP-B210 obtain FDA 510k
2016.12.15
The fistula home-care model, KP-B210 successfully completes the 510k registration of US FDA. Since Dec 2016, it’s legally available in US. Please contact us for further enquiry.
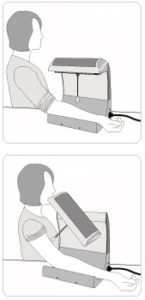
More product information, please click here.
